Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect

Resorcinarene‐Based o‐Biarylphosphines in Palladium‐Catalysed Suzuki–Miyaura Cross‐Coupling Reactions of Bulky Substrates - Elaieb - 2017 - European Journal of Inorganic Chemistry - Wiley Online Library

China Tetrakis(triphenylphosphine)palladium(0) (Pd(PPh3)4) CAS No.: 14221-01-3 Manufacturers - Free Sample - Alfa Chemical

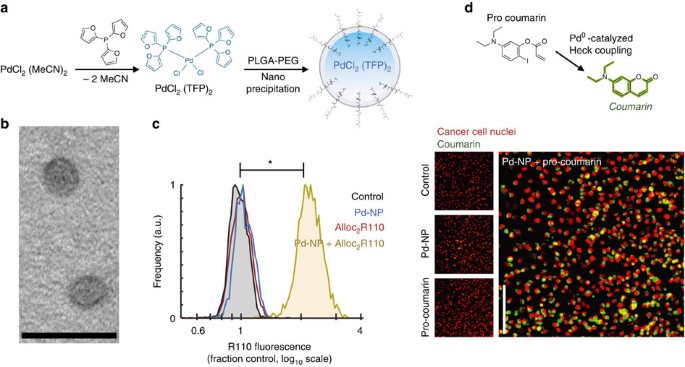
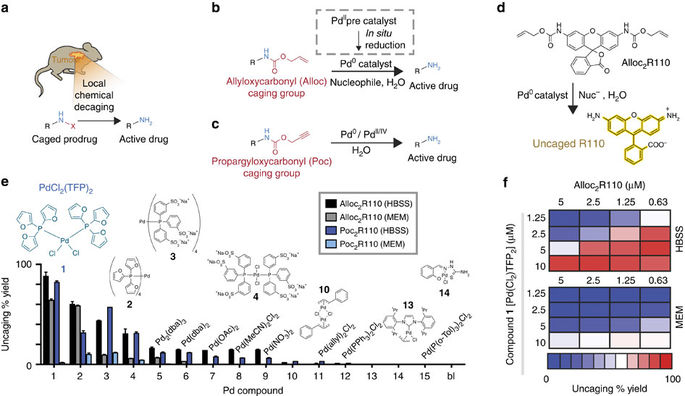
Activation of AllylUE (1) and PropUE (2) probes in presence of Pd or Pt... | Download Scientific Diagram
![Tris[tri(2‐thienyl)phosphine]palladium as the catalyst precursor for thiophene‐based Suzuki‐Miyaura crosscoupling and polycondensation - Li - 2008 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library Tris[tri(2‐thienyl)phosphine]palladium as the catalyst precursor for thiophene‐based Suzuki‐Miyaura crosscoupling and polycondensation - Li - 2008 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library](https://onlinelibrary.wiley.com/cms/asset/4dbbd39f-04c1-40c9-be16-0a73bbc6236d/mgra001.jpg)
Tris[tri(2‐thienyl)phosphine]palladium as the catalyst precursor for thiophene‐based Suzuki‐Miyaura crosscoupling and polycondensation - Li - 2008 - Journal of Polymer Science Part A: Polymer Chemistry - Wiley Online Library
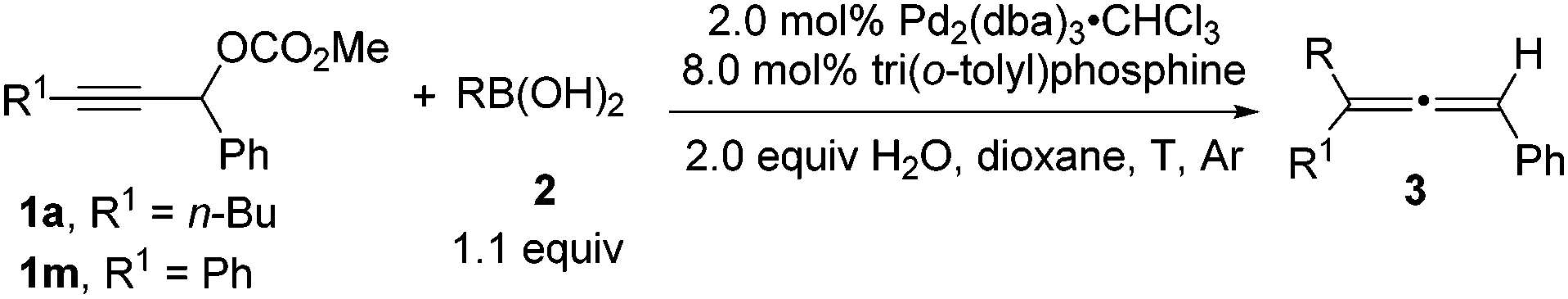
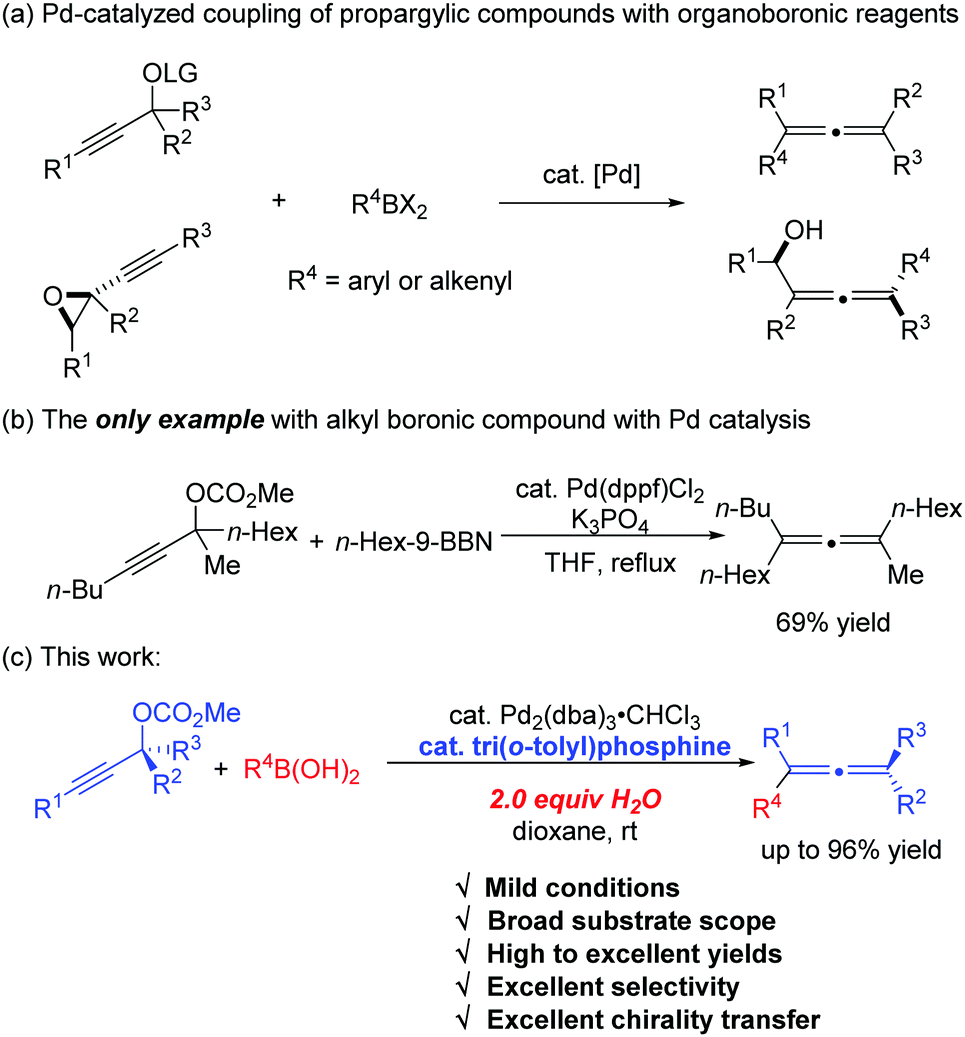
Tri(o-tolyl)phosphine for highly efficient Suzuki coupling of propargylic carbonates with boronic acids - Chemical Communications (RSC Publishing)
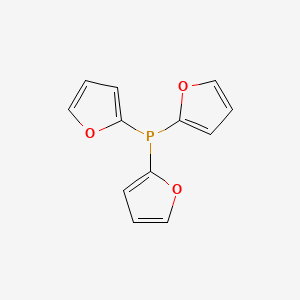
The preparation, resolution and application of novel 2-furyl phosphine ligands in asymmetric synthesis

Carbonylation of terminal alkynes catalysed by Pd complexes in combination with tri(2-furyl)phosphine and methanesulfonic acid - ScienceDirect
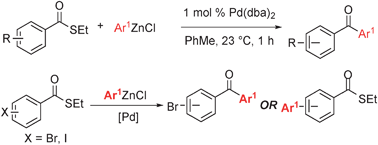
Synthesis of diaryl ketonesvia a phosphine-free Fukuyama reaction - Chemical Communications (RSC Publishing)