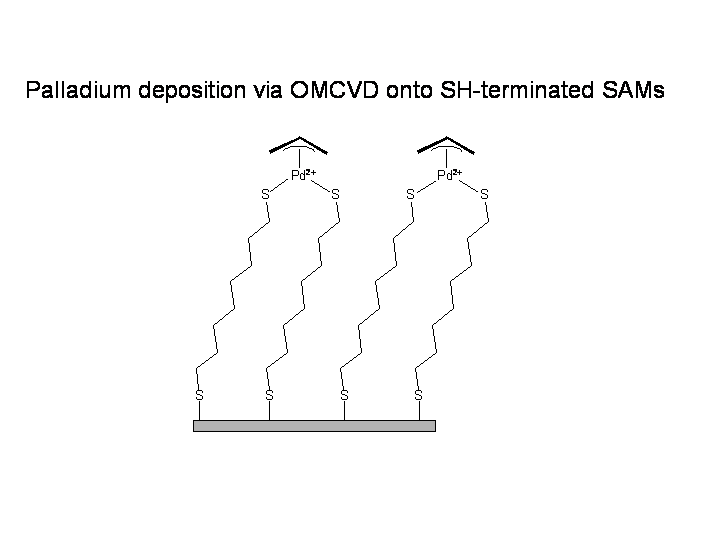
Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -
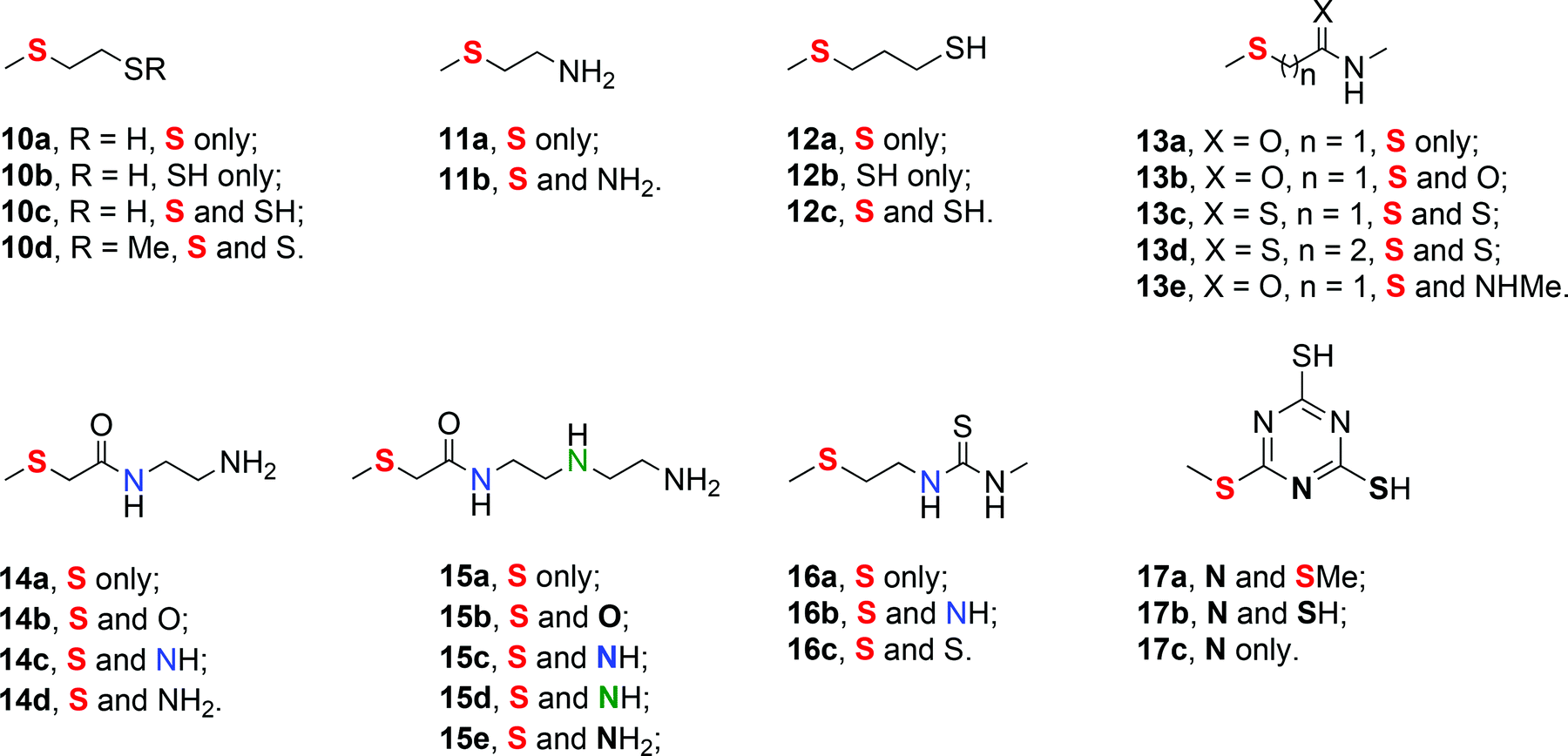
Towards a quantitative understanding of palladium metal scavenger performance: an electronic structure calculation approach - Dalton Transactions (RSC Publishing) DOI:10.1039/C3DT52282B

Palladium prompted on-demand cysteine chemistry for the synthesis of challenging and uniquely modified proteins | Nature Communications
Towards a quantitative understanding of palladium metal scavenger performance: an electronic structure calculation approach - Dalton Transactions (RSC Publishing) DOI:10.1039/C3DT52282B

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect
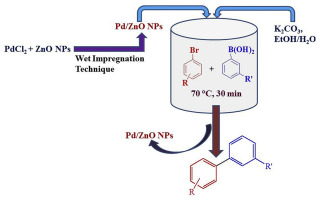
Palladium loaded on ZnO nanoparticles: Synthesis, characterization and application as heterogeneous catalyst for Suzuki–Miyaura cross-coupling reactions under ambient and ligand-free conditions - Mater. Chem. Phys. - X-MOL

Thiol Functionalized Cross-Linked Chitosan Polymer Supporting Palladium for Oxidative Heck Reaction and Reduction of p-Nitrophenol | SpringerLink

Reusable and Flexible Heterogeneous Catalyst for Reduction of TNT by Pd Nanocube Decorated ZnO Nanolayers onto Electrospun Polymeric Nanofibers - Arslan - 2017 - ChemistrySelect - Wiley Online Library

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect

Immobilization of Pd nanoparticles on the surface of thiol-modified MWCNTs | Download Scientific Diagram

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect

Thiol Treatment Creates Selective Palladium Catalysts for Semihydrogenation of Internal Alkynes - ScienceDirect
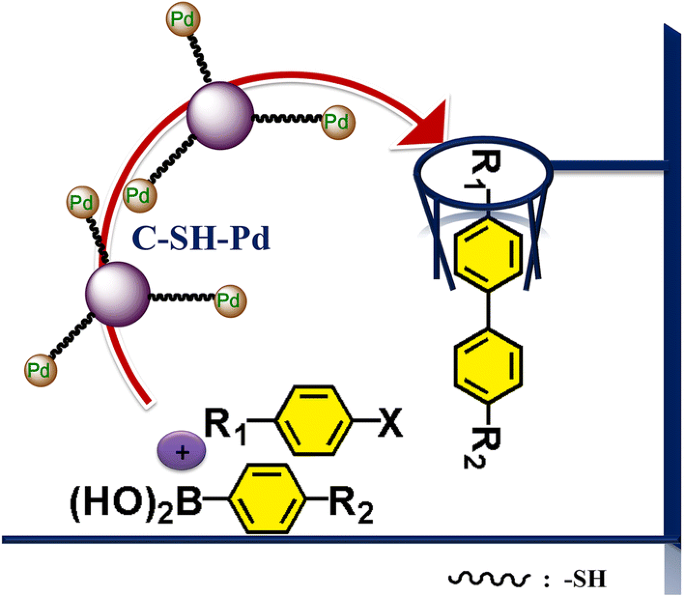
Palladium Nanoparticles Anchored on Thiol Functionalized Xylose Hydrochar Microspheres: An Efficient Heterogeneous Catalyst for Suzuki Cross-Coupling Reactions | SpringerLink
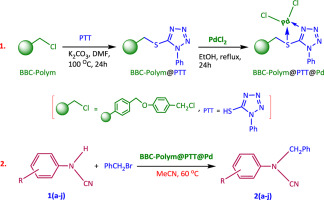
A novel tetrazole functionalized polymer-supported palladium nano-catalyst for the synthesis of various N-benzylated arylcyanamides - J. Alloys Compd. - X-MOL